
Foreign Involvement
Foreign Involvement & International Engagement
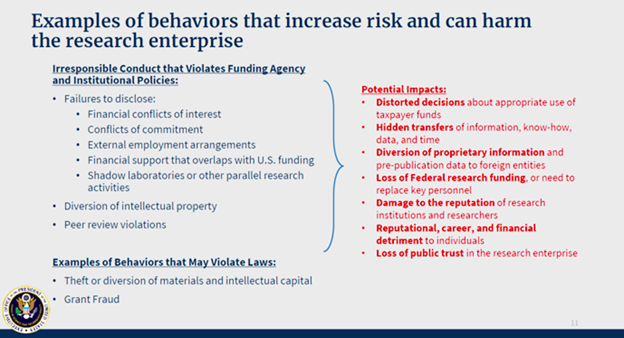
The White House Office of Science and Technology Policy (OSTP) provides some examples of behaviors that can increase risk and harm the research enterprise.
Guidance for the NSU Research Community
Nova Southeastern University and all other US educational institutions must comply with federal reporting and disclosure requirements. Below you will find general guidance regarding the types of relationships and activities that NSU researchers are expected to disclose, as part of existing university and federal funding requirements. Please note that each Federal sponsor has their own specific guidance, much of which is currently being revised.
NSU researchers should ensure they disclose all applicable “Other Support” as required by federal sponsors. “Other Support” may include financial resources, domestic or foreign, available in direct support of a researcher’s research endeavors. Such support should be disclosed on an “Other Support” or “Current & Pending” form.
To help ensure full transparency and disclosure of foreign and domestic research activities, federal agencies have recently revised, or are revising, their guidance and/or requirements for disclosing sources of research support via Other Support or Current & Pending Support. The Office of Sponsored Programs has a created a website to provide general guidance to NSU investigators for completing Other Support, as well as information on specific federal requirements, which vary slightly across different agencies. It is ultimately the responsibility of the individual researcher to ensure that the report of Other Support (Current & Pending Support) is complete and accurate to the best of their knowledge.
- NSU Guidance on Other Support (Current & Pending)
- NIH Policies on Other Support and on Policies related to Financial Conflicts of Interest and Foreign Components
- NIH Requirements for Disclosure of Other Support, Foreign Relationships and Activities, as well as Conflicts of Interest
- NIH Other Support guidance
- NIH Disclosure Table (includes guidance on both Other Support and Biographical Sketch*)
- COGR Commentary on Disclosing Other Support
- NSF Current and Pending Support Instructions
- Table: Disclosures Required in your Biographical Sketch and Current/Pending (Other Support), (updated May 2024)
- DoD memo on disclosing other support(March 2019)
Investigators and other key personnel are also required to disclose on their Biographical Sketch any academic professional, or institutional appointments, whether or not remuneration is received, whether the appointment is with a foreign or domestic organization, and whether the appointment is full-time, part-time or voluntary. Sponsor and program requirements can vary, so it is important for each individual to review and follow the specific instructions of the funding agency, as published in their agency policies and or funding application instructions. If you have questions regarding these requirements, please reach out to the Office of Sponsored Requirements.
- NIH Disclosure Table (includes guidance on both Other Support and Biographical Sketch*)
- NIH Biosketch Format Pages, Instructions and Samples
- Table: Disclosures Required in your Biographical Sketch and Current/Pending (Other Support), (updated May 2024)
- NSF Information for Creating a Biosketch (information on policy, FAQ’s and using Science Experts Network Curriculum Vitae SciENcv)
Foreign components of federally funded research must be disclosed on proposals, progress reports, and final technical reports. NIH defines a Foreign Component as
the performance of any significant scientific element or segment of a project outside of the United States, either by the recipient or by a researcher employed by a foreign organization, whether or not grant funds are expended. Activities that would meet this definition include, but are not limited to, (1) the involvement of human subjects or animals, (2) extensive foreign travel by recipient project staff for the purpose of data collection, surveying, sampling, and similar activities, or (3) any activity of the recipient that may have an impact on U.S. foreign policy through involvement in the affairs or environment of a foreign country.
On 08/15/2024, the NIH published a decision matrix for assessing potential foreign interference as part of its ongoing efforts to be transparent about its policies and procedures. The decision matrix builds on the detailed information already available on their site, including the process they take to handle new allegations of foreign interference, and also offers additional detail as to how NIH considers whether to contact institutions to request additional information. NIH strongly supports properly conducted and principled international collaborations that are integral for our country to remain competitive.
Other sponsors have similar requirements to disclose foreign components.There are multiple ways in which foreign components can be disclosed, e.g.,
- Identifying a “foreign component” in a grant application;
- Listing a “non-U.S. performance site”;
- Identifying foreign relationships and activities in a biosketch (see above);
Principal Investigators (PIs) should review all pending proposals and active awards to ensure that all foreign components have been disclosed.
The CHIPS and Science Act of 2022 (Public Law 117-167) directs federal research sponsors to impose certain prohibitions and requirements related to “foreign talent recruitment programs” that affect NSU investigators on sponsored projects. Information about these programs and the associated prohibitions and requirements for disclosure/certification is provided below.
Definitions:
Foreign Talent Recruitment Program (FTRP): Any program, position, or activity that includes compensation in the form of cash, in-kind compensation, including research funding, promised future compensation, complimentary foreign travel, things of non de minimis value, honorific titles, career advancement opportunities, or other types of remuneration or consideration directly provided by a foreign country at any level (national, provincial, or local) or their designee, or an entity based in, funded by, or affiliated with a foreign country, whether or not directly sponsored by the foreign country, to an individual, whether directly or indirectly stated in the arrangement, contract, or other documentation at issue.
Malign Foreign Talent Recruitment Program (MFTRP): (reference White House Office for Science and Technology Memorandum February 14, 2024 for complete definition): An MFRTP is an FTRP (as defined above) that is sponsored by a foreign country of concern (per Section 10612 of PL 117-167), by an entity based in a foreign country of concern, or by an institute or program on a restricted list (in accordance with section 1286(c)(9) of PL 115-232).
An MFTRP also includes any program, position or activity that involves or incorporates one or more of the following:
- Engaging in unauthorized transfer of intellectual property, materials, data or other non-public information owned by a U.S. entity
- Requiring recruitment of other trainees/researchers to enroll in such a program, position or activity;
- Establishing a lab or taking any type of position or appointment that violates federal research award terms and conditions
- Being unable to terminate the activity except in extraordinary circumstances
- Engaging in work that creates overlap or duplication with federally funded research
- Requiring application for and receipt of funding from foreign government funding agencies
- Requiring omission of acknowledgements of U.S. university affiliation or of federal funders
- Requiring non-disclosure to NSU or to federal funders regarding involvement in the program, position or activity, or
- Having a conflict of interest/commitment that does not comply with the federal research award
Prohibition: Participation in a MFTRP by NSU principal investigators and key research personnel is prohibited by the Chips and Science Act and the associated implementing rules of federal agencies.
Disclosure Requirements: Investigators must disclose participation in any FTRP in accordance with federal requirements through the avenues of disclosure presented herein.
Certifications: As part of NSU’s internal proposal approval process in Cayuse, investigators must certify they have made appropriate disclosures, and that they are not participating in a MFTRP. Additionally, federal agencies are also requiring individual certifications by covered personnel on their Other Support and Biographical Sketches as part of the federal application process.
For Guidance: If you need assistance determining whether an activity or relationship may be considered a foreign talent recruitment program, contact researchsecurity@nova.edu. If you have questions about the proper mechanism of disclosure, contact fcoi@nova.edu.
Certain financial interests in a foreign entity, including governments and universities, must be disclosed to NSU in accordance with applicable federal, state, and NSU requirements.
In accordance with Federal regulations, NSU has a responsibility to manage, reduce, or eliminate any actual or potential conflicts of interest that may be present in a Study Team. OSP Policy #16, Financial Conflicts of Interest in Sponsored Programs , is intended to meet the federal requirements governing disclosure, management and reporting of financial conflicts of interest (FCOI's).
Export control regulations are federal laws that restrict the export of specific items, information, and software for reasons related to U.S. national security, economic and foreign policy goals. NSU is committed to complying with all U.S. export control laws in both research and non-research activities.
Export controls usually arise for one or more of the following reasons:
- The nature of the export has actual or potential military applications or economic protection issues
- Government concerns about the destination country, organization, or individual, and
- Government concerns about the declared or suspected end use or the end user of the export
NSU’s Export Control website offers additional information regarding the NSU Export Control Policy, applicability, exclusions and exemptions. Please contact exportcontrol@nova.edu with any questions.
The federal government has shared concerns about improperly safeguarding certain types of privileged information. Through the peer review process, faculty may have access to confidential information or Intellectual Property in grant applications. NIH peer review policies strictly prohibit sharing an application with anyone who has not been officially designated as a reviewer.
Individuals researching dual-use technology and/or sensitive emerging technologies should be especially cautious about safeguarding confidential information or Intellectual Property. Additionally, research on dual-use technologies and/or emerging technologies should be reviewed for possible export control implications, so please contact exportcontrol@nova.edu.
- If you have question or concerns about safeguarding your data and devices or require a secure computing environment for your research, please contact the Office of Innovation and Information (OIIT). You can find OIIT’s policies and procedures on Information Security at https://www.nova.edu/portal/oiit/policies/index.html. If the research is funded by an outside agency or organization, the funding agreement might have additional data security requirements, and we will work with you and OIIT to ensure the obligations are met.
- If a faculty, staff, or student believe that their research has resulted in an invention that is novel and has possible commercial value, they should submit a confidential Invention and Disclosure form to the NSU Office of Technology Transfer.
- Please refer to NSU’s OSP Policy on Research Data Ownership, Management, Retention and Access for more information on how to protect the NSU’s investment in research.
University faculty and staff routinely host international colleagues for short-term visits to campus. In rare cases, visiting scholars may be pressured by their home countries to inappropriately access information or research facilities and equipment.
If you are asked to facilitate the visit of a foreign delegation or asked to sign a Memorandum of Understanding with a foreign institution, please contact the Office of International Students and Scholars (OISS). OISS administers NSU’s Exchange Visitor Program and will provide guidance and assistance in hosting a foreign scholar. If you intend for an international visitor to participate in research activities, please contact exportcontrol@nova.edu in addition.
Currently, all international travel must receive the college Dean’s approval, and then the approval of either the Provost or COO of Nova Southeastern University. Once this approval is received in writing, you may begin the additional travel registration as required by the Office of International Affairs. Additionally, if you plan to take any device (including NSU laptops), NSU data, or NSU equipment, please contact exportcontrol@nova.edu. Travel to certain countries may restrict travel with these items.
Travel to sanctioned or embargoed countries and other countries with heightened security requirements require review by International SOS and secondary authorizations will be required.
Please note that trips to Level 4 or 5 destinations will require secondary authorization from your department’s Dean or Vice President.
Agency-Specific Links & Statutes
- National Institutes of Health Protecting U.S. Biomedical Intellectual Innovation
- Department of Defense Foreign Influence Letter
- Department of Energy Foreign Government Sponsored or Affiliated Activities
- Department of Energy Foreign Government Talent Recruitment Programs
- Research Security at the National Science Foundation
- DoD - Basic Research/Research Directorate –Academic Research Security
- CHIPS and Science Act of 2022, Section 10631, 10632
- William M. (Mac) Thornberry National Defense Authorization Act for Fiscal Year 2021, Section 223(a)1
- White House Office for Science and Technology Memo